BANG! Why Are Buffers Important To The Human Body
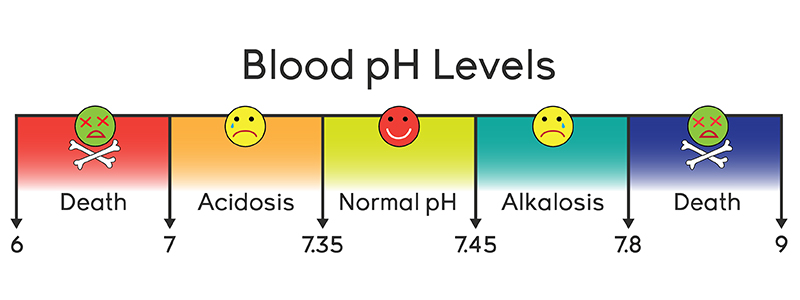
A variety of buffering systems exist in the body that helps maintain the pH of the blood and other fluids within a narrow rangebetween pH 735 and 745. The human body has one of the most complicated and effective buffer systems ever known to the mankind and collectively these systems are responsible for maintaining human life.
The Buffer System In The Blood Animation Annenberg Learner
The protein buffer system is an integral component of the bodys pH controlling mechanism.

Why are buffers important to the human body. Buffering system of blood When any acidic substance enters the bloodstream the bicarbonate ions neutralize the hydronium ions forming carbonic acid and water. Buffer solutions are important because they help to neutralize a reaction to a certain extent. What 6 elements make up 95 of the body weight of organisms CHNOPS carbon hydrogen nitrogen oxygen phosphorus and sulfur.
Rxns reactions in the body happen take place In the blood plasma and these reactions might fail to happen if the blood pH keeps changing. 3 are much greater than concentration of H ions. If hydrogen ion increases then it combines with the buffer if it decreases some hydrogen ions.
Other body organs play important roles in this buffer system. Why is it important to have buffers in blood. Reactions in human body.
To maintain pH homeostasis. This means that changes in the concentration of H ions have little effect on the pH of blood. For a complete rxn to take place the.
Therefore buffers are commonly used in living organisms to help maintain a relatively stable pH. What are buffers and why are they important to life. A buffer is a chemical substance that helps maintain a relatively constant pH in a solution even in the face of addition of acids or bases.
Acidic buffers are used to neutralize alkaline solutions because of the weak acids in the alkaline solution. The lungs get rid of most. Most diseases illnesses and bad bacteria thrive in an over acidic environment.
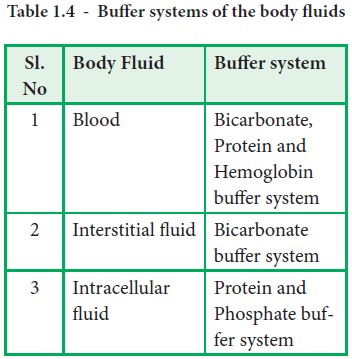
The three major buffer systems of our body are carbonic acid bicarbonate buffer system phosphate buffer system and protein buffer system. In general a buffer is a solution that contains substances which has the ability to minimize changes in the pH when acids or bases are added to the solution. Protein buffers are either intracellular or extracellular.
The body also has a buffer system that helps control the level of acids and bases 1. Carbonic acid bicarbonate buffer system. The most important physiological buffers in the body are the bicarbonateCO 2 system the large anion complexes such as plasma proteins and phosphates and hemoglobin in cells.
Cellular respiration produces carbon dioxide as a waste product. How do buffers work in the human body. Buffer solutions are important because they help to neutralize a reaction to a certain extent.
There are many reasons why buffers are important in the body. The carbonatecarbonic acid buffer the phosphate buffer and the buffering of plasma proteins. The metabolism in the human body produces high amounts of acids.
This is hydrolysed into bicarbonate ion in the blood. Buffer solutions are important functions. In humans for example buffers act to maintain blood pH between 735 and 745 even though acids and bases are continually being added to and removed from the blood as it travels through the body.
Most biochemical reactions that are essential for life only take place in a narrow pH range. A buffer system in the human body is an interaction between a weak acid-base conjugate pair that keeps the body at. This buffer works well because concentrations of the buffer components HCO.
Buffers are an important part of the biochemical processes of living things because they help keep the pH within organisms body stable. It is important for us to assist our body in creating and maintaining a healthy pH ratio of 7030 alkaline to acid. PH levels are controlled using bicarbonate or HCO3-.
This causes the body to borrow crucial minerals from organs bones and. Make an organism suited to its way of life. While in the blood this bicarbonate ion serves to neutralise acids introduced in to the blood.
The three major buffer systems of our body are carbonic acid bicarbonate buffer system phosphate buffer system and protein buffer system. The presence of buffers ensures that the bodys pH remains in this range despite changes in the surroundings. The three major buffer systems of our body are carbonic acid bicarbonate buffer system phosphate buffer system and protein buffer system.
Why are buffers important to the human body. 2 formed from H. The bodys chemical buffer system consists of three individual buffers out of which the carbonic acid - bicarbonate buffer is considered most important.
Buffering is important in living systems as a means of maintaining a fairly constant internal environment also known as homeostasis. This buffer system is essential because exercise produces carbon dioxide and lactic acid in muscles. What is important of adaptations.
Buffers are important because it keeps the pH steady and within normal limits in organisms. PH tolerances vary by body system but in every single case its incredibly important to maintain it due to its undesirable effects like the denaturing of proteins. While the third buffer is the most plentiful the first is usually considered the most important since it is coupled to the respiratory system.
Why are buffer solutions important to the body. Read more about facts about human body pH levels. An important buffer system in the human body is the bicarbonate buffering system that keeps human blood in the right pH range.
When pH levels are unbalanced it is mostly in the case of being too acidic. An example of. Proteins are the most important and widely operating buffers in the body fluid.
A buffer is a chemical substance that helps maintain a relatively constant pH in a solution even in the face of addition of acids or bases. Buffers are chemicals that reduce major pH changes in your body fluids blood intracellular fluid interstitial fluid. The bodys chemical buffer system consists of three individual buffers.
The carbonic acid-bicarbonate buffer system plays an extremely important role in maintaining pH homeostasis of the blood. In all of these the essential reaction is. A buffer which contains an acid and its conjugate base or a base and its conjugate acid is capable of offsetting the introduction of an undesirable quantity of either an.
Why are buffers important to the human body.

Buffers What Are The Importance Of Buffers In Biological System

Acid Base Homeostasis Flashcards Quizlet

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Ph And Buffer System In Body Fluids

Chemical Buffer Systems And Acid Base Balance
Buffer Systems In The Human Body By Matthew Siino

Which Of The Following Is Not One Of The Body S Chemical Buffering Systems Lifeder English
Buffers What Are The Importance Of Buffers In Biological System

Chemical Buffers Protein Buffer Phosphate Buffer System And Bicarbonate Buffer System Youtube
Buffers In The Blood By Georgia Bloodworth
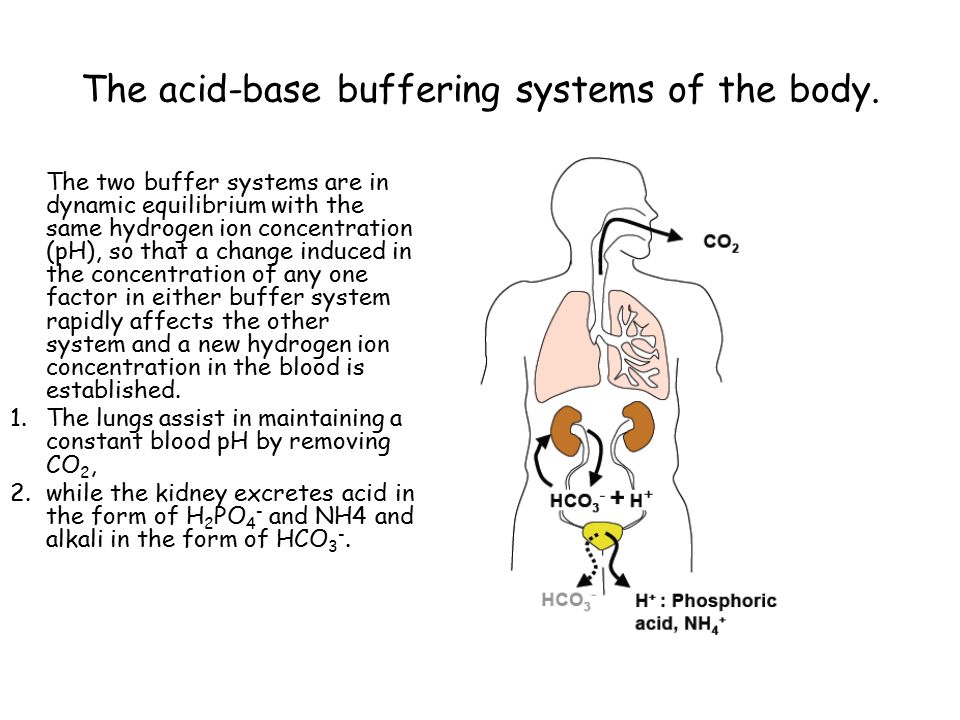
Acid Base Balance And Buffer Systems In The Human Body Ppt Video Online Download

Buffer Action In The Blood Youtube

The Major Body Buffer Systems Download Table

The Major Body Buffer Systems Download Table

Acid Base Biochemistry Flashcards Quizlet

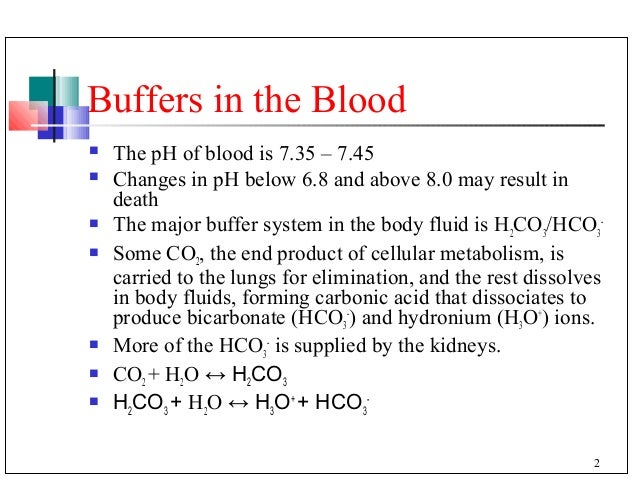
Comments
Post a Comment